Medi-Mani | GP-RPM
GP-MDAI – Golden Phoenix Healthcare Technologies
Remote Patient Monitoring (RPM) is a type of telemedicine that uses technology to monitor a patient's health from a remote location. RPM systems typically include a device, such as a smart watch, that a patient wears and tracks their vital signs, such as heart rate, blood pressure, and blood sugar levels. The device transmits the data to a healthcare provider, who can then review the data and provide feedback or adjust medications as needed. RPM can be used to monitor chronic conditions, such as diabetes, or acute conditions, such as post-surgical recovery. It can also be used to monitor the health of elderly patients or those with limited mobility. RPM has been found to be an effective way to monitor a patient's health, reduce hospital readmissions, and improve patient outcomes.
According to March 2022 report from Markets and Markets, the global remote patient monitoring market in terms of revenue was estimated to be worth $53.6 billion in 2022 and is poised to reach $175.2 billion by 2027.
The GP-RPM™ device is an AI-based RPM that connects by AI from head to heart to get your key 6+ vital sign and 6 health indicators including heart rate, blood pressure, oxygen saturation level, HRV-SDNN, stress index and respiration rate using NIH-originated remote photoplethysmography and remote ballistocardiography methods.

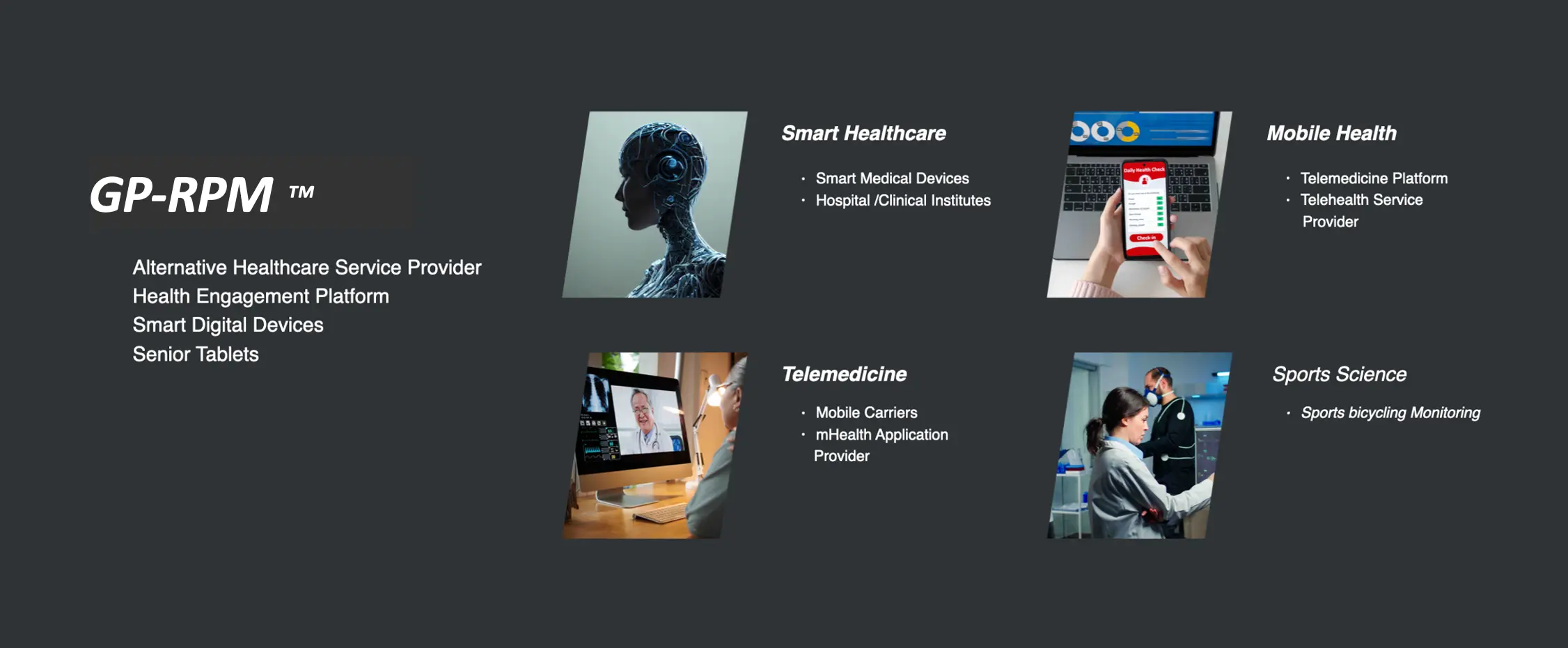
Centers for Medicare and Medicaid Services (CMS) pays healthcare providers for using RPM systems provided to qualified patients in the United States. CMS's 2021 Final Rule stated that RPM services are limited to “established patients.” However, for the duration of the COVID-19 public health emergency (PHE), CMS waived the “established patient” requirement and allowed practitioners to bill for RPM for new patients. Our GP-RPM™ device can be applied in four healthcare sectors encompassing Smart Healthcare, Mobile Health, Telemedicine, and Sports Science.
Given Medicare’s reimbursement availability for RPM, Medicare Advantage Plans, and Accountable Care Organizations (ACO), a data analysis has been conducted, applying the double-blind randomized controlled study of more than 30 million data points using Philip’s continuous monitoring devices for 3000+ patients, which helps measure accuracy >95% of 6 health indicators. FDA application has been applied and anticipating approval in Q2 2023, where it will be the first FDA-approved contactless RPM without using any ancillary devices for measuring 6 vital signs. With our existing deep rooted relationships among US national healthcare networks along with existing scalable BPOs for RPM, we can then bring the true RPM values to the elderly population in mass.
Contact
Contact Us
Location:
2375 E Camelback Rd, #600B, Phoenix, AZ 85016
Email Us:
Call Us:
+1 866-686-2284

